FDA bans BPA in baby bottles and sippy cups
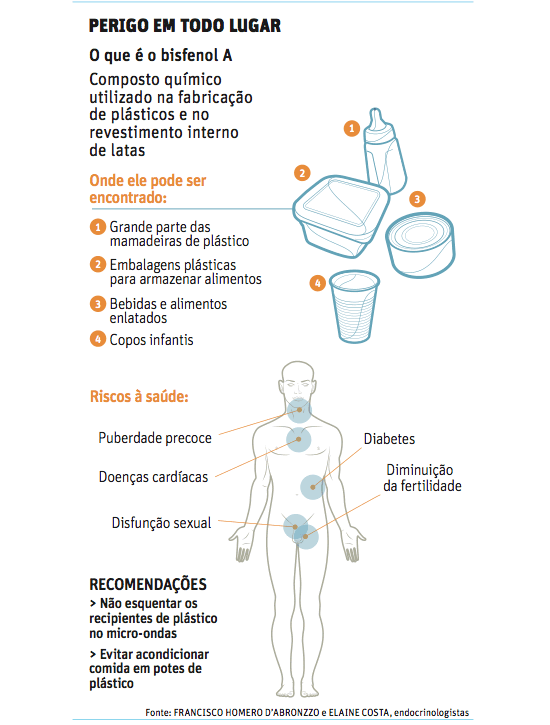
Bisfenol. Infográfico Correio Braziliense
It was an easy call; nobody makes these any more By Janet Raloff, Science News
From now on, U.S. manufacturers may no longer produce polycarbonate baby bottles and sippy cups (for toddlers) if the clear plastic had been manufactured from bisphenol A, a hormone-mimicking compound. Long-awaited, the announcement is anything but a bold gesture. The Obama administration decided to lock this barn door after the cow had died.
In the July 17 Federal Register, the U.S. Food and Drug Administration announced that effective immediately, it will no longer permit BPA-based polycarbonate products aimed specifically at feeding children. Its justification: production of these goods for the U.S. market “has been completely and permanently abandoned.”
In fact, the American Chemistry Council, a trade group representing (among others) plastics manufacturers, petitioned for the action. Presumably, member companies that have voluntarily phased out the controversial formulation don’t want to risk any foreign hold outs encroaching on their territory. But whatever ACC’s motivation, acknowledges San Francisco-based Sarah Janssen, a senior public health scientist with NRDC, an environmental advocacy group, FDA’s action on BPA-based products is a step in the right direction.
Albeit a quite-limited one.
Plenty of youngsters still drink from polycarbonate tumblers. Parents chop and mix up plenty of meals using food processors that invariably have a polycarbonate bowl. Many moms dispense beverages from polycarbonate pitchers and bring polycarbonate flatware to picnics and other use-and-toss events. So kids still face a substantial opportunity for exposure to BPA (which can leach from the plastic).
Plastic kitchenware is not the only dietary source of BPA exposures, however. Resins made from BPA — and able to leach the environmental hormone — line a large share of metal food cans. FDA made no move to prohibit this use. And that’s why Rep. Edward Markey (Dem.-Mass.) on March 16 drafted three separate petitions to FDA. They asked the agency to phase out the use of BPA-based materials in baby-food and infant-formula containers, in canned food and beverages and in small reusable food and beverage containers (like sports bottles, tumblers and boxes for storing food leftovers).
FDA rejected the petitions, arguing that since manufacturers haven’t yet abandoned these uses, it couldn’t unilaterally prohibit them (at least promptly). So Markey moved on one front where BPA-based resins have been phased out: the packaging for infant formula. A proposal asking FDA to ban BPA-based materials in this former application also appears in the July 17 Federal Register.
But even if FDA were to move on BPA for this or other products, there’s still a host of related compounds standing poised in the wings as willing understudies. Chief among them: bisphenol S — a functional analog for bisphenol A in some products, most notably in thermal receipt paper.
As we reported several weeks back, European Commission scientists have just released preliminary data indicating that BPS possesses a hormonal alter ego every bit as worrisome as BPA’s. And prompting that government study: concern about whether BPS would be any safer than BPA for use in baby bottles. Clearly, if companies haven’t begun selling “BPA-free” bottles made from BPS, it’s under discussion.
“Right now, BPS is most widely used in those paper products that once relied on BPA (like receipt paper),” observes Alex Formuzis of the Environmental Working Group, a Washington-based advocacy organization that has taken a strong stand against BPA exposures in children. “But infant formula makers and the food and beverage industry could make the switch [to BPS] tomorrow,” he says. Acting against BPA does nothing to prohibit another untested or largely unstudied alternate from be substituted.
Finally, leaving BPA-based resins and plastics on the market allows adult exposure. Maturity may reduce susceptibility to damage from this hormone mimic but not eliminate it altogether, animal studies indicate. Moreover, any exposures sustained by pregnant women may allow a developing fetus to share in her dose. In fact, animal data suggest exposures in the womb may pose the greatest, most long-lasting risks from these materials.
I subscribe to the precautionary principle, which is why I buy insurance, take my car in for routine servicing and get an annual mammogram. Perhaps it’s time we apply the precautionary principle to hormone-mimicking pollutants as well. It seems downright foolhardy to countenance their continued use in products expressly designed to contact anyone’s food or drink.
EcoDebate, 18/07/2012
[ O conteúdo do EcoDebate é “Copyleft”, podendo ser copiado, reproduzido e/ou distribuído, desde que seja dado crédito ao autor, ao Ecodebate e, se for o caso, à fonte primária da informação ]
Inclusão na lista de distribuição do Boletim Diário do Portal EcoDebate
Caso queira ser incluído(a) na lista de distribuição de nosso boletim diário, basta clicar no LINK e preencher o formulário de inscrição. O seu e-mail será incluído e você receberá uma mensagem solicitando que confirme a inscrição.
O EcoDebate não pratica SPAM e a exigência de confirmação do e-mail de origem visa evitar que seu e-mail seja incluído indevidamente por terceiros.